From Ancient Greece to the Atomic Nucleus
colosieve
Ancient Greece: The Birth of Atomic Theory
Around 440 BCE, Greek philosopher Democritus[^1] proposed a radical idea:
“Everything is composed of tiny, indivisible particles called atomos (ἄτομος) — meaning ‘uncuttable’ or ‘indivisible’.”
The concept:
- Matter cannot be divided infinitely
- Eventually you reach fundamental, indivisible units
- These atoms are eternal, unchangeable, and invisible
- Different arrangements create different substances
The problem:
- Pure philosophy—no experiments, no evidence
- Rejected by Aristotle (who favored continuous matter)
- Forgotten for over 2,000 years

Preamble: Electrons Discovered (1897)
J.J. Thomson[^2] discovers electrons using cathode ray tubes—the first subatomic particle ever identified.
What it proved:
- Atoms weren’t indivisible after all (Democritus was wrong about that)
- But atoms DO exist as fundamental building blocks (Democritus was right about that)
The new question: If electrons exist inside atoms, what does the atom actually look like?


Thomson’s “Plum Pudding” Model (1904)
Thomson proposed: an atom is like a plum pudding[^3]
- A blob of positive charge (the “pudding”)
- Tiny negative electrons embedded throughout (the “plums”)
- The positive charge is evenly spread across the entire atom
Result: This seemed reasonable. Everyone accepted it.

Brownian Motion (1827)
77 years earlier…
Botanist Robert Brown[^4] looked at pollen grains floating in water under a microscope.
What he saw: The grains were jiggling around randomly, constantly, for no apparent reason.
The mystery: No one could explain why. The puzzle sat unsolved for 78 years.
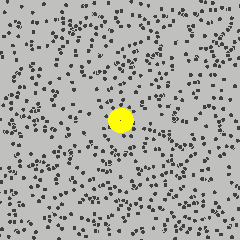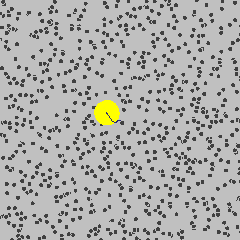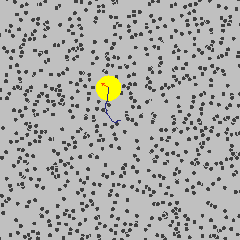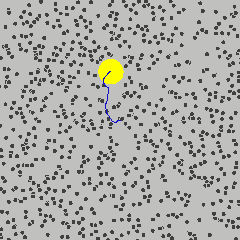
Einstein’s Breakthrough (1905)
Albert Einstein[^5] solved it.
His explanation:
- Water molecules are real
- They’re constantly moving
- They’re constantly bombarding the pollen grain from all sides
- The jiggling you see IS the proof
The impact:
- He gave precise mathematical predictions
- Experiments confirmed them
- This finally convinced the last skeptics: atoms were real

Discovery of the Atomic Nucleus (1909-1911)
Now that atoms were proven real, what was inside them?
Ernest Rutherford[^6] and his team fired alpha particles[^7] (tiny, positively charged bullets) at a super-thin sheet of gold foil—only a few atoms thick.
This experiment would change everything.

The Gold Foil Experiment
The Setup:
- Alpha particles: Positively charged particles from radioactive decay (helium nuclei)
- Shot from a radioactive source through a narrow slit
- Aimed at ultra-thin gold foil (only a few atoms thick)
- Detected by a fluorescent screen that flashes when hit
What they expected (based on plum pudding model):
- Particles would pass straight through
- Like bullets through tissue paper
- The positive charge was spread out, so nothing should stop them
What actually happened:
- Most particles passed through
- But some bounced straight back

Rutherford’s Shock
Rutherford later said:
“It was quite the most incredible event that has ever happened to me in my life. It was almost as incredible as if you fired a 15-inch shell at a piece of tissue paper and it came back and hit you.”
What It Meant
The atom doesn’t have evenly spread positive charge.
Instead:
- There’s a tiny, incredibly dense, positively charged nucleus at the center
- Almost all the atom’s mass is packed into that tiny core
- The electrons orbit around it
- Most of the atom is empty space
Result:
- The plum pudding model was dead
- The nuclear model was born
The Thread
Ancient Greece (~440 BCE): → Democritus proposes atoms are indivisible particles
“Do atoms even exist?” (1827-1905) → Brown observes mysterious motion; Einstein proves atoms are real
“What are they made of?” (1897) → Thomson discovers electrons—atoms ARE divisible
“What’s inside them?” (1904) → Plum pudding model: evenly distributed positive charge
The shocking truth (1909-1911): → A tiny, dense nucleus revealed by gold foil experiment
The lesson: Most of the atom is empty space, with all the mass concentrated in the center.
